The Valvezan laboratory studies the complex interplay between the signaling pathways and metabolic networks that control cell growth in physiological and disease states. The large-scale biosynthetic programs that underlie cell growth and proliferation are strongly influenced by signaling pathways that activate metabolic networks in response to growth-promoting cues. We are defining the exact nature and extent of metabolic control by specific signaling pathways, along with the underlying molecular mechanisms that link signaling to metabolism.
Although large-scale metabolic reprogramming is a ubiquitous hallmark of cancer cells, activating mutations or amplifications of metabolic enzymes themselves are rare in cancer. Instead, cancer cells typically stimulate anabolism by co-opting the signaling nodes that control large metabolic networks. This allows cancer cells to coordinately upregulate many metabolic pathways that must function cooperatively to produce complex biomass, rather than upregulating a single metabolic enzyme or pathway in isolation. Many of the most common oncogenic driver mutations uncouple these signaling and metabolic outputs from their normal regulatory inputs. Although this provides a growth advantage, locking pathways in the “on” state can come at the cost of reduced plasticity and increased dependence on specific nutrients, enzymes or pathways for sustained growth and viability. Thus the uniquely reprogrammed metabolic networks in cancer cells offer opportunities to identify and target metabolic processes that are uniquely essential in those cells. To exploit these “metabolic vulnerabilities”, we seek to understand how signaling pathways coordinate metabolic networks, and the impact of common oncogenic mutations. By leveraging novel insights in these areas, we work to identify unique cancer dependencies that can be translated into new therapeutic approaches.
Current projects include:
Targeting ribonucleotide (NTP) synthesis pathways to selectively kill cancer cells
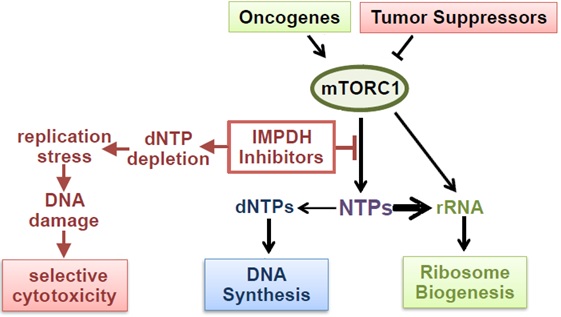
Maintaining intracellular nucleotide pools is essential for cell proliferation and viability. We discovered that tumor cells with high rates of ribosomal RNA (rRNA) synthesis are uniquely dependent on nucleotide synthesis pathways to maintain their free nucleotide pools and sustain rapid rRNA and
DNA synthesis (Valvezan et al., 2017, Cancer Cell). This metabolic dependency can be exploited using clinically approved inhibitors of the rate-limiting enzyme in de novo guanylate nucleotide synthesis (IMPDH), which cause nucleotide depletion and apoptosis selectively in tumor cells with elevated rRNA synthesis rates. In cell and tumor models of the genetic tumor syndrome Tuberous Sclerosis Complex (TSC), IMPDH inhibitors demonstrate strong anti-tumor efficacy at clinically relevant doses (Valvezan et al., 2017, Cancer Cell; Valvezan et al., 2020, JCI Insight).
TSC tumors are driven by uncontrolled activation of the master metabolic regulator mTOR Complex 1 (mTORC1). We discovered that uncontrolled mTORC1 activation confers dependence on nucleotide synthesis pathways by strongly driving rRNA synthesis. As mTORC1 is activated in the majority of human tumors across nearly all lineages, we are expanding these nucleotide synthesis inhibitor studies to determine the full translational potential of this targetable metabolic vulnerability. IMPDH inhibitors have been used clinically for decades as safe and well-tolerated immunosuppressants, but are not currently used as anti-tumor agents. By contrast, direct inhibitors of dNTP synthesis and DNA synthesis have been used as chemotherapeutic agents for decades, but are associated with significant toxicity which can limit their efficacy. By targeting NTP synthesis rather than dNTP/DNA synthesis, IMPDH inhibitors have increased specificity for targeting tumor cells with high rates of rRNA synthesis, which corresponds to their markedly reduced toxicity profiles in patients. The use of clinically approved IMPDH inhibitors in our studies means that efficacy could lead to rapid repurposing for cancer patients.
Defining and targeting metabolic reprogramming in colorectal cancer
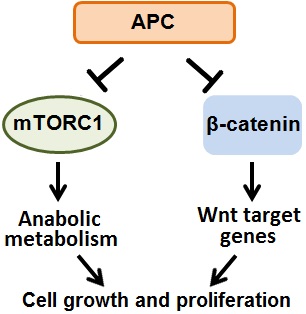
Colorectal cancer is the 2nd leading cause of cancer-related death in the United States, and is responsible for over 800,000 deaths globally each year. Sporadic truncating mutations in the APC tumor suppressor gene promote the onset of 80% of human colorectal cancers, and germline APC mutations cause the intestinal polyposis and colorectal cancer predisposition syndrome Familial Adenomatous Polyposis, with 100% penetrance. Thus it is imperative to understand the role of APC in cellular signaling and metabolism, and the consequences of APC mutations in colorectal cancer. However, despite their potent tumorigenic effects, very little is known about how APC mutations alter cellular metabolism. APC mutations activate the canonical Wnt/β-catenin signaling pathway and in parallel also activate the master metabolic regulator mTOR Complex 1. We are defining the metabolic consequences of APC mutations in colorectal cancer with the goal of identifying and exploiting unique metabolic dependences. Intestinal nutrients and the microbiota provide a particularly interesting and complex microenvironment compared to many other solid tumors which are exclusively fed by serum metabolites. Thus we are also determining how the cell intrinsic effects of APC mutations and the intestinal tumor microenvironment interact to produce the complex metabolic phenotype of colorectal cancer cells.
Defining cellular stresses sensed by mTOR Complex 1 to identify tumor vulnerabilities
Under physiological conditions, the master metabolic regulator mTOR Complex 1 integrates growth factor, nutrient, and energy signals to stimulate anabolic cell growth only when sufficient cellular resources are available. Conversely, mTORC1 is inhibited when cellular resources are limited, or in response to various stresses. This adaptive response conserves cellular resources and prevents mTORC1 from continuing to drive its anabolic program, which could exacerbate a stressed state. Oncogenic mutations activate mTORC1 by uncoupling it from these upstream regulatory signals. This drives anabolic pathways to support uncontrolled tumor cell growth, but can also render cells unable to properly respond to specific stresses. We are identifying cellular stresses sensed by mTORC1 to unveil novel tumor vulnerabilities. The failure of tumor cells with uncontrolled mTORC1 activity to respond appropriately to specific stresses could allow selective targeting of those cells through pharmacological induction of such stresses.
Read More › about Alexandra Grument received 2 very prestigious awards at Rutgers University
Read More › about Ariel Lerner accepted into Mount Sinai Icahn School of Medicine.
Read More › about mTORC1 activity oscillates throughout the cell cycle, promoting mitotic entry and differentially influencing autophagy induction
Read More › about Alexander Valvezan awarded NJCCR 2022 Pediatric Research Grant
Read More › about Alexander Valvezan receives Breast Cancer Alliance Young Investigator Award
Read More › about Alexander Valvezan receives Leukemia Research Foundation Award for project titled, “Repurposing IMPDH inhibitors for selective targeting of PTEN-deficient T-ALL cells”
Read More › about Conversations with CABM Director: An interview with Dr. Alexander Valvezan
Read More › about Assistant Professor Alexander Valvezan is the 2020 recipient of a Rutgers Cancer Institute of New Jersey New Investigator Award
Read More › about CABM welcomes Alexander Valvezan as a resident faculty member







